Open position for a PhD student candidate in the Glauser lab
Analysis of nociception and avoidance behaviours in Caenorhabditis elegans
Avoidance behaviours are crucial for survival in virtually every animal and rely on nociception, the processes of detecting noxious stimuli and transmitting them in the nervous system. In humans, nociceptive processes are also responsible for pain sensation. Pain is an important medical concern and its satisfactory management is an unmet need in modern medicine. Any advance in our understanding of the molecular substrates, cellular processes, or the neural circuits in the nociceptive pathway may help developing better therapeutic approaches for pain management.
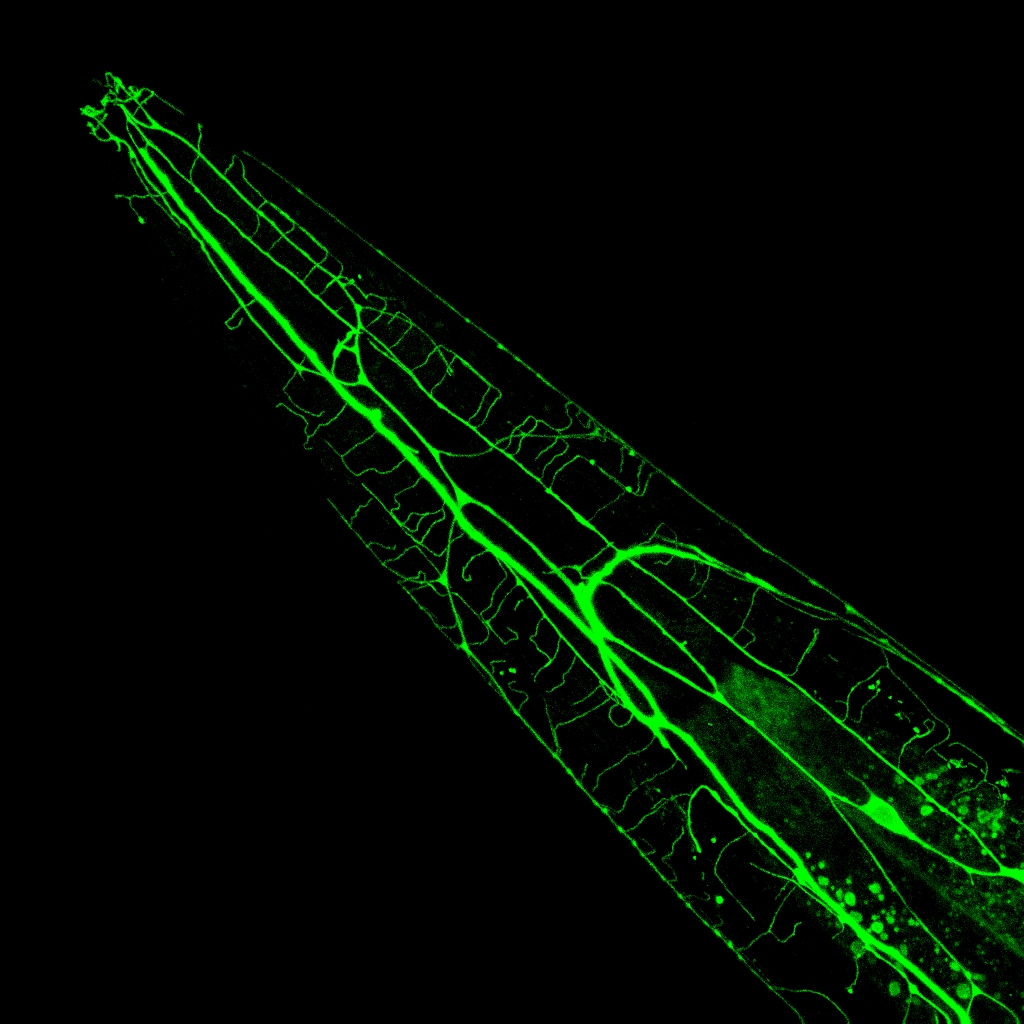
The research on nociception in human and mammalian models is hindered by the size and complexity of their nervous systems, and the paucity of available genetic methods. In contrast, the nematode Caenorhabditis elegans displays a number of advantages, like a fast growth, powerful genetic tools a transparent body and a compact nervous system composed of exactly 302 neurons, whose synaptic connectivity has been fully mapped. Furthermore, the worm shares nearly half of its genes with humans, and there is a large conservation of the molecular pathways controlling neural functions
-
Nociception genes
In order to learn more about the molecular basis of nociception, our starting point is to identify and characterize genes, which are required for avoidance behaviors. To that end, we perform forward and reverse genetic screens using different behavioural assays. Most avoidance genes that we identified so far have orthologues in humans. For example, we found that that the nociceptive function of Transient Receptor Potential Vanilloid (TRPV) proteins is conserved between C. elegans and humans (Glauser et al. 2011). Thus, by accelerating the discovery of additional molecular and cellular signalling mechanisms that control the function of neurons in the nociceptive pathways, our research in C. elegans has strong potential medical implications.
-
Nociception plasticity
While worm avoidance behaviours are robust innate responses that are essential for survival, they are nevertheless modulated by past experience. For example, prolonged exposure to noxious temperatures causes desensitization and a subsequent decrease in noxious heat avoidance. We have discovered new molecular mechanisms controlling this plasticity. We found that the sensitivity of nociceptor neurons is regulated by the controlled subcellular localization of Calcium/calmodulin-dependent Protein Kinase-1 (CMK-1), (Schild et al., 2014). CMK-1 re-localizes from the cytoplasm to the nucleus during nociceptor neuron desensitization, and its nuclear activity is both necessary and sufficient to reduce the output delivered by the cell and to inhibit the resulting aversive behavior. This signal transduction “switch” provocatively suggests ways to produce analgesia, and unquestionably calls for deeper investigations.
-
Toward an integrated view of nociception and its plasticity
One of our main current goals is to use the simple C. elegans model to obtain a comprehensive view of the complex mechanisms controlling nociception and its plasticity; and this at the molecular, cellular, circuit and behavioural levels. To that end, we use a combination of cutting-edge techniques including in vivo neuron activity imaging, opto- and chemo-genetics, tissue-specific transcriptomics, proteomics and phosphoproteomics, as well as high-content computer-assisted behavioral analyses.
As a whole, our research paradigm with C. elegans provides a pipeline for the rapid identification and extensive characterization of potentially conserved genes involved in nociception and its plasticity, which may provide new tracks to develop better pain therapeutic strategies.