Boris August Egger
Head of Bioimage Core Facility Lecturer in Light...
PER 05 - 0.340G
+41 26 300 8881
E-mail
During brain development neural stem cells go through phases of cell proliferation followed by phases of differentiation. Symmetric proliferative cell divisions lead to a rapid increase in the stem cell pool while in asymmetric divisions stem cells are self-renewed and at the same time more specialized cell types are generated. The transition between division modes needs to be tighly regulated as excessive symmetric cell division can lead to tumorous overgrowth whereas precocious asymmetric division might lead to premature differentiation and underdeveloped organs.
In our research we use genetics and confocal microscopy to observe the cellular and molecular mechanisms that control the transition of stem cell states in the fruit fly model Drosophila melanogaster. We focus on two main questions:
• how transcription factors and signalling pathways coordinate the transition of neural stem cell states?
• how environmental factors such as oxygen control neural stem cell homeostasis and differentiation?
We have recently shown in collaboration with Simon Sprecher's group that the nuclear hormone transcription factor Tailless (Tll) has a crucial role in regulating the maintenance of symmetrically dividing neuroepithelial stem cells (Guillermin et al., 2015). Mutations in the tailless gene result in cell death of neuroepithelial cells, failure in the specification of progenitor cells and the loss of neurons in all optic brain ganglia of the developing visual system. Since Tll/TLX transcription factors are evolutionary highly conserved between flies and mammals our findings are relevant to gain insights into neural stem cell state transitions during our own brain development.
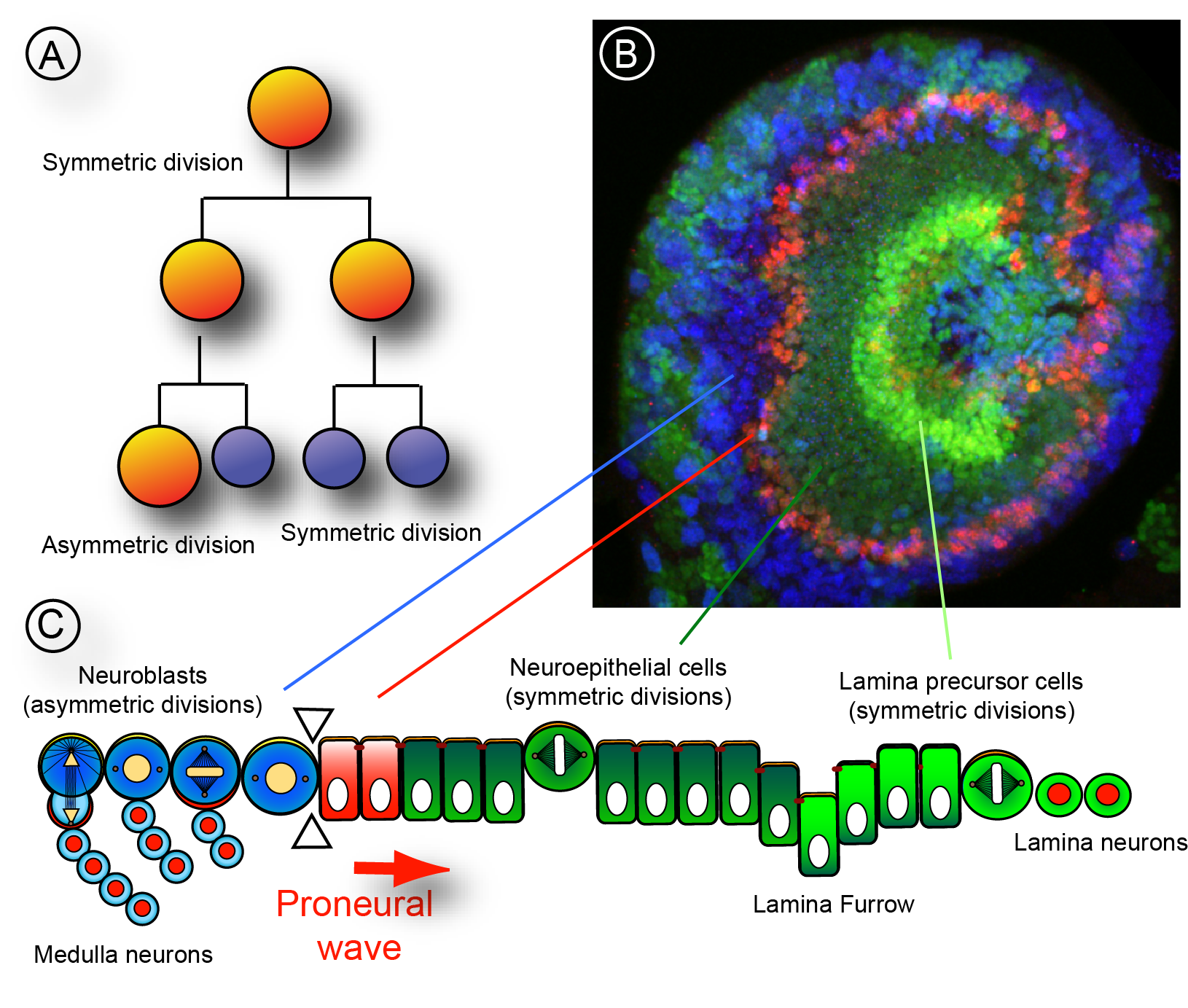
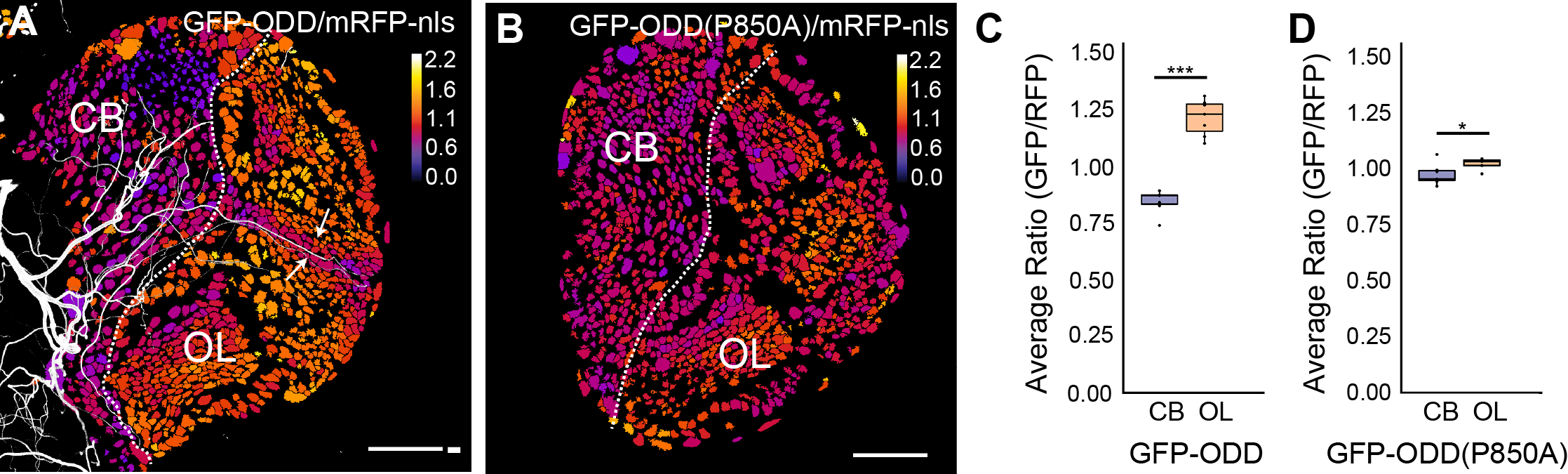
Neural stem cell homeostasis in the nervous system is not only regulated through intrinsic molecular signalling pathways but also through signals that emanate from surrounding tissues. Stem cells are often found in niches, specific regions in a tissue that provide the microenvironmental cues that regulate stem cell proliferation, self-renewal and differentiation. Oxygen is one such critical signal that can affect stem cell properties under normal tissue homeostasis or under disease condition such as tumour formation. Interestingly, low oxygen levels, or hypoxia, are a hallmark of stem cell niches and of proliferating tumour cells. Therefore oxygen supply needs to be developmentally regulated and oxygen signalling might be co-ordinated with other signalling pathways during neural tissue homeostasis. As in other insects, efficient gas supply is mediated in Drosophila by a network of trachea, air-filled tubes connected to the exterior space through small openings. Gas exchange occurs between the thinnest respiratory tubes, called tracheoles, and surrounding cells. We use the Drosophila larval brain as a model system to investigate how oxygen supply in proliferating neural tissue is regulated and how oxygen signalling affects the states of neuroepithelial cells and neuroblast.
In collaboration with Stefan Luschnig's and Rafael Cantera's group we recently developed a fluorescent biosensor and an imaging pipeline to analyse the hypoxic state of neural progenitor cells (Misra et al., 2016).
Our bioimage laboratory is continously working on new methods to improve protocols for live imaging of Drosophila primary cell culture and whole mount larval brain samples.
Head of Bioimage Core Facility Lecturer in Light...
PER 05 - 0.340G
+41 26 300 8881
E-mail